
 |
| A dilution factor named "e" |
|
Some people may estimate that the final concentration in A will be 0.5 g/L,
corresponding to a lowering of the original concentration of 1 g/L
by a factor of 2. But the dilution factor is 2.71828 . Those who had never used e in practice did it just now. The name "e" was given by Leonard Euler in 1748. e appears in cases where a lot of very small steps are used, in particular an indefinite sum of indefinite small steps. The term "continuous" is used for such a process and continuous dilution is a very graphic example. The final concentration after filling an equal-sized vessel B is 1/e = 0.368. The next figure shows a model with two vessels B and C to be filled: |
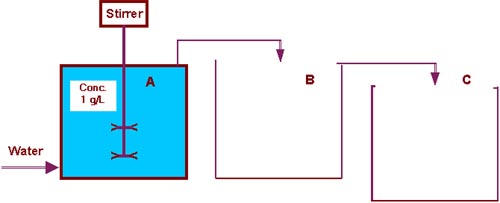
|
| Vessels | Repeated dilution |
Total dilution factor |
c formula |
c (c0 = 1) | |
| 1 | 1 /e | e1 | 2.72 | c0 * e-1 | 0.368 |
| 2 | 1 /e /e | e2 | 7.39 | c0 * e-2 | 0.135 |
| 3 | 1 /e /e /e | e3 | 20.09 | c0 * e-3 | 0.050 |
| 4 | 1 /e /e /e /e | e4 | 54.60 | c0 * e-4 | 0.018 |
| 5 | 1 /e /e /e /e /e | e5 | 148.41 | c0 * e-5 | 0.007 |
| 6 | ......... | ||||
 |
Detour |
 |